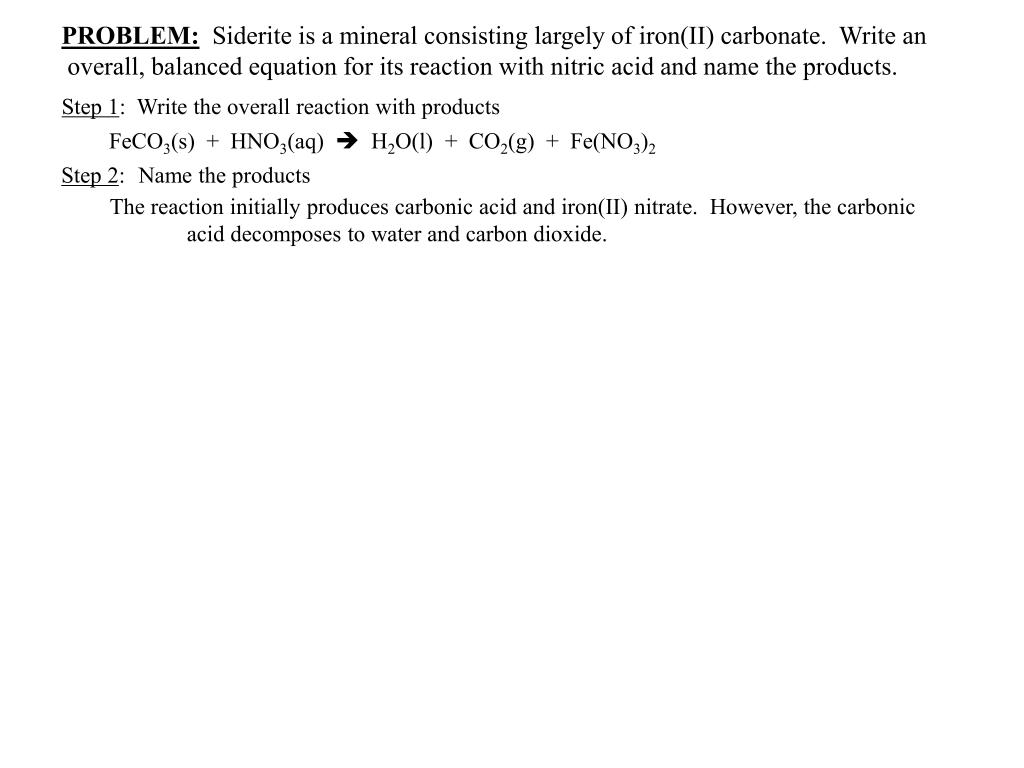
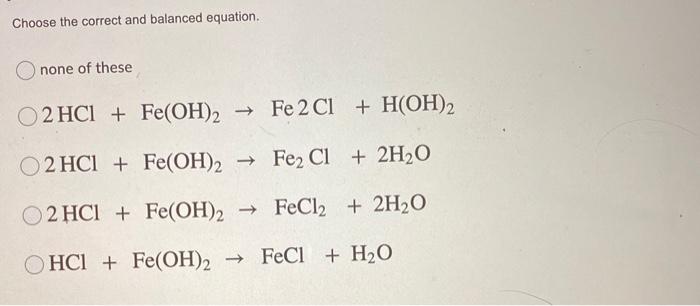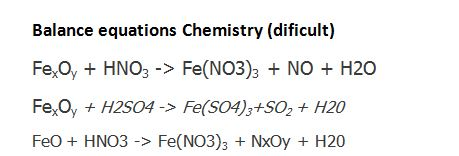SOLVED: Question 33 (2 points) When the following equation is balanced, what is the coefficient folllkz Fe HNO3 Fe(NO3)3 Hz 0
![A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com](https://homework.study.com/cimages/multimages/16/2_redox_balancing_11041037245791770796.png)
A. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? [ ? ] F e 2 + + [ ? ] C I O 2 ? [ ? ] F e + [ ? ] C I O 3 ? B. Water appe | Homework.Study.com
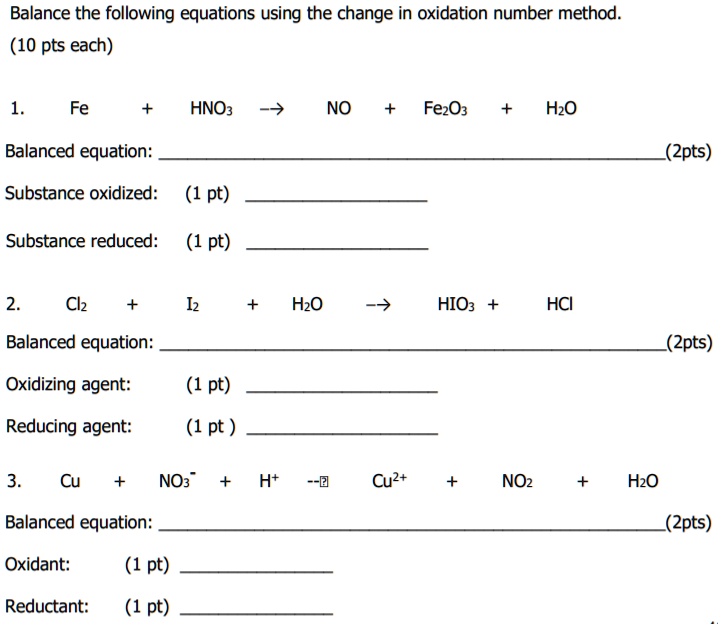
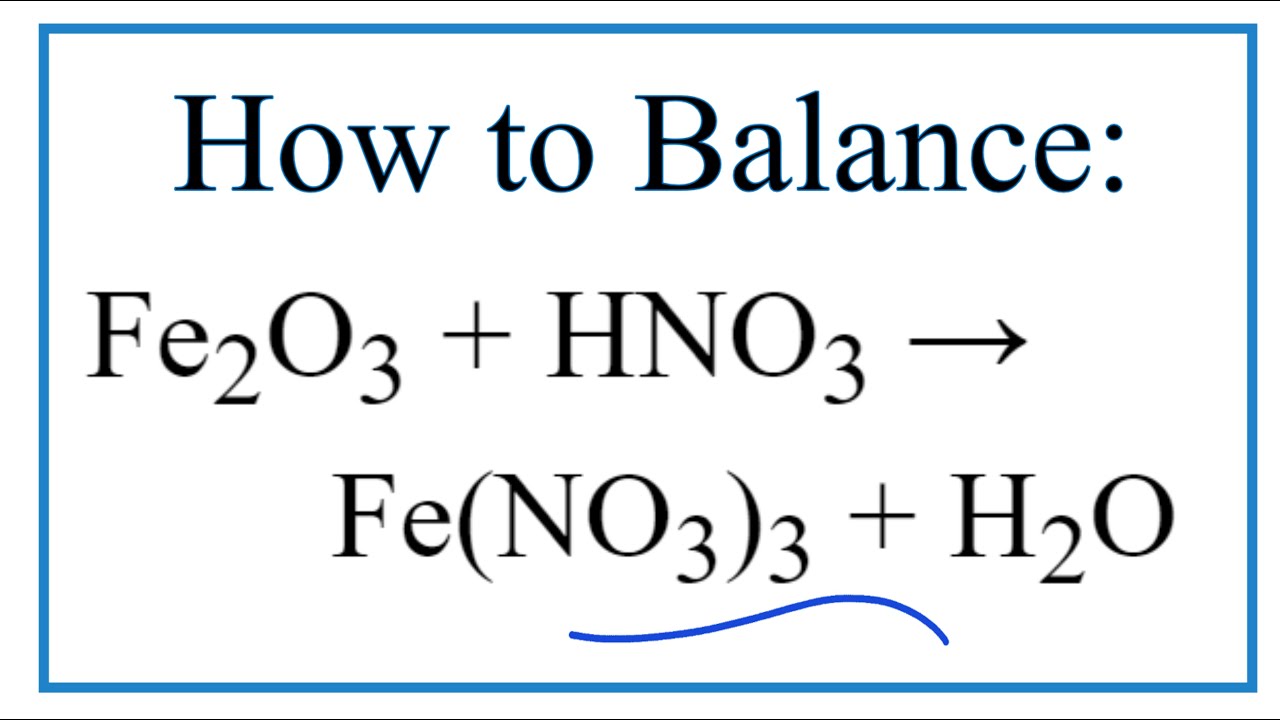
SOLVED: Balance the following equations using the change in oxidation number method (10 pts each) Fe HNOz NO FezOz Hzo Balanced equation: (Zpts) Substance oxidized: (1 pt) Substance reduced: (1 pt) Clz
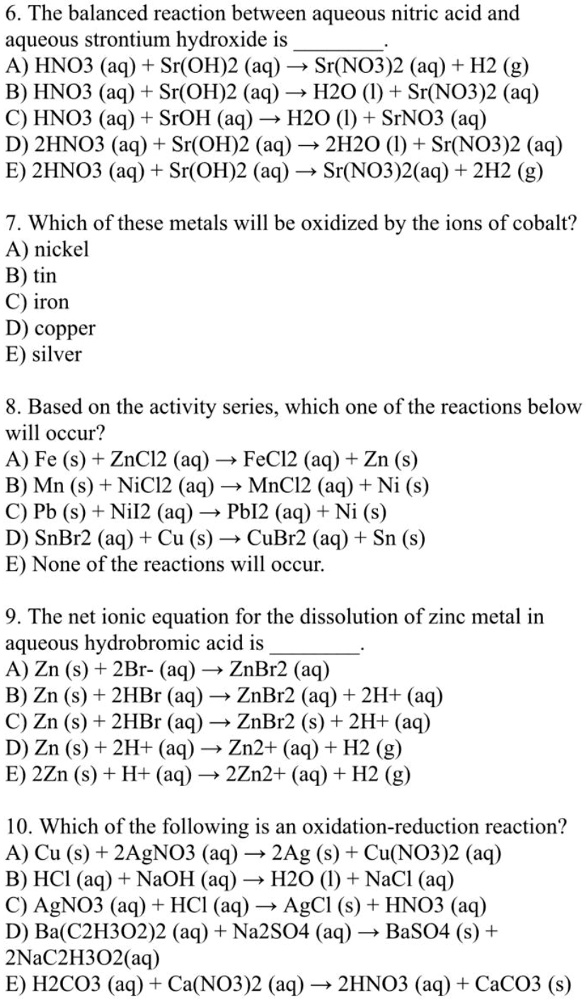
SOLVED: The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is HNO3 (aq) + Sr(OH)2 (aq) 57 Sr(NO3)2 (aq) + H2 (g) B) HNO3 (aq) + Sr(OH)2 (aq) 5 H2O (